How Do You Know Which Lewis Structure Is the Best
If you look on the periodic table you will notice that H has one valence electron C has 4 and N has 5. This chemistry video provides a basic introduction into how to draw lewis structures of common molecules such as Cl2 O2 OF2 CH4 NH3 H2O C2H2 and N2H4.

No2 Lewis Structure Nitrite Ion Lewis Math Molecules
LEWIS STRUCTURES General Rules for Drawing Lewis Structures 1.

. If its the former the explanation is below. One way to do this is to write the Lewis symbols for all of the atoms in the formula and count up all the dots. Formal chargeThe charge assigned to an atom in a molecule assuming that electrons in a chemical bond are shared equally between atoms.
When counting electrons negative ions should have extra electrons placed in their Lewis structures. Indicate which N-O bonds are 118 pm and which N-O bonds are 136 pm in the bonding framework below. Provide the best Lewis structure by drawing in any missing multiple bonds and lone pairs.
You may need a periodic table for this. Typically the structure with the most charges on the atoms closest to zero is the more stable Lewis structure. Draw the Lewis Structure.
This type of Lewis dot structure is represented by an atomic symbol and a series of dots. Octet ruleAtoms lose gain or share electrons in order to have a full valence shell of eight electrons. When answering questions that involve resonance structures it is important to take into account all of the resonance structures and not only the.
Draw the Lewis Dot Structure for the Hydrogen atom. Determine the total number of valenceelectrons. 118 pm and 136 pm.
The lewis structure with formal charges closest to 0 is best in the sense that makes a bigger contribution to the resonance hybrid than the other two resonance structures as is stated on page 73 of the course reader. Write the Lewis dot structure for the molecule. See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding.
Therefore if you are. An atom molecule or ion has a formal charge of zero if it has the number of bonds that is typical for that species. However as we go down the group the atomic sizes increase which helps to handle the negative charge more efficiently because the charge density decreases with large volumesurface.
If we have three F atoms that means that we are. Assume that you must determine the bond angles in BF3. Positive ions should have fewer electrons than an uncharged molecule.
So the best Lewis structure has a NO double bond with two lone pairs on oxygen and a lone pair and one unpaired electron on nitrogen. For a molecule uncharged that count is the correct number of valence electrons. When the Lewis structure of an ion is written the entire structure is placed in brackets and the charge is written as a superscript on the upper right outside of the brackets.
To draw the Lewis structure for HCN we will first calculate the total number of valence electrons. Start with the components of the molecule atoms polyatomics and count the valence electrons available then place them where they need to go to fulfill octet charge balance and electro negativity preferences. This helps determine which of a few Lewis structures is most correct.
Write the skeleton structure of the molecule. All valence electrons of the atoms in Lewis structures must be shown. The Lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs.
This observation works best only when the two atoms bearing the formal charge are in the same row of the periodic table since they have comparable atomic sizes. Begin drawing the Lewis dot structure of the molecule. Now lets apply the above rules to predict the best Lewis structure for the molecule Hydrogen Cyanide HCN.
Its essential for predicting molecular geometry molecule polarity and reactivity in a compound. You do not need to provide the Lewis structure again. According to my 9th grade textbook a Lewis structure is just a Kekule structure except the bonds are.
First of all a correct count of all valence electrons is essential. Unpaired electrons are observed in odd electron molecules such as NO and NO2. Lewis dot structures can be drawn to show the valence electrons that surround an atom itself.
Use two valence electrons to form each bond inthe skeleton structure. Need help with chemistry. If it is the latter sodium then its because this is an ionic compound and we build the lewis structures of the cation and anion separately.
First and foremost carbon EN25 is less electronegative than nitrogen EN31 so by your rule it should be in the center. Try to satisfy the octets of the atoms bydistributing the remaining valence electrons as nonbondingelectrons. As described on page 73 of the course reading the lewis structure with formal charges closest to 0 is best in the sense that it makes a greater contribution to the resonance mixture than the other two resonance structures.
How do you know which Lewis structure is the most stable. The N-O bond lengths in dinitrogen pentoxide have two values. B is less electronegative than F so B becomes the central atom.
Generally electrons are paired.

How To Draw Lewis Structures Youtube

Best Lewis Structure Practice Youtube

Lewis Structure Of Po3 1 Simple Method For Lewis Electron Dot Structures Context Clues Worksheets Lewis High School Chemistry

Brf3 Lewis Structure Bromine Trifluoride

Lewis Structures Introduction Formal Charge Molecular Geometry Resonance Polar Or Nonpolar Youtube

N3 Lewis Structure Azide Ion Ap Chemistry Molecules Lewis
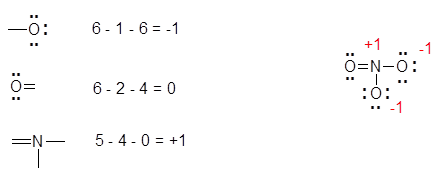
Lewis Structures Chemistry Libretexts

Drawing Lewis Structures Resonance Structures Chemistry Tutorial Youtube Chemistry Science Education Organic Chemistry

How To Draw A Lewis Structure Lewis College Life Hacks Middle School Science

Lewis Structures Introduction Formal Charge Molecular Geometry Resonance Polar Or Nonpolar Youtube Molecular Geometry Molecular Chemistry

7 3 Lewis Symbols And Structures Chemistry

Lewis Structures Expanded Octet Pcl5 Janet Coonce Takes Time To Draw This Diagram Step By Step A Great Help High School Science Secondary Science Chemistry

From Gen Chem To Org Chem Pt 7 Lewis Structures Master Organic Chemistry Organic Chemistry Molecular Geometry Organic Chemistry Study
Comments
Post a Comment